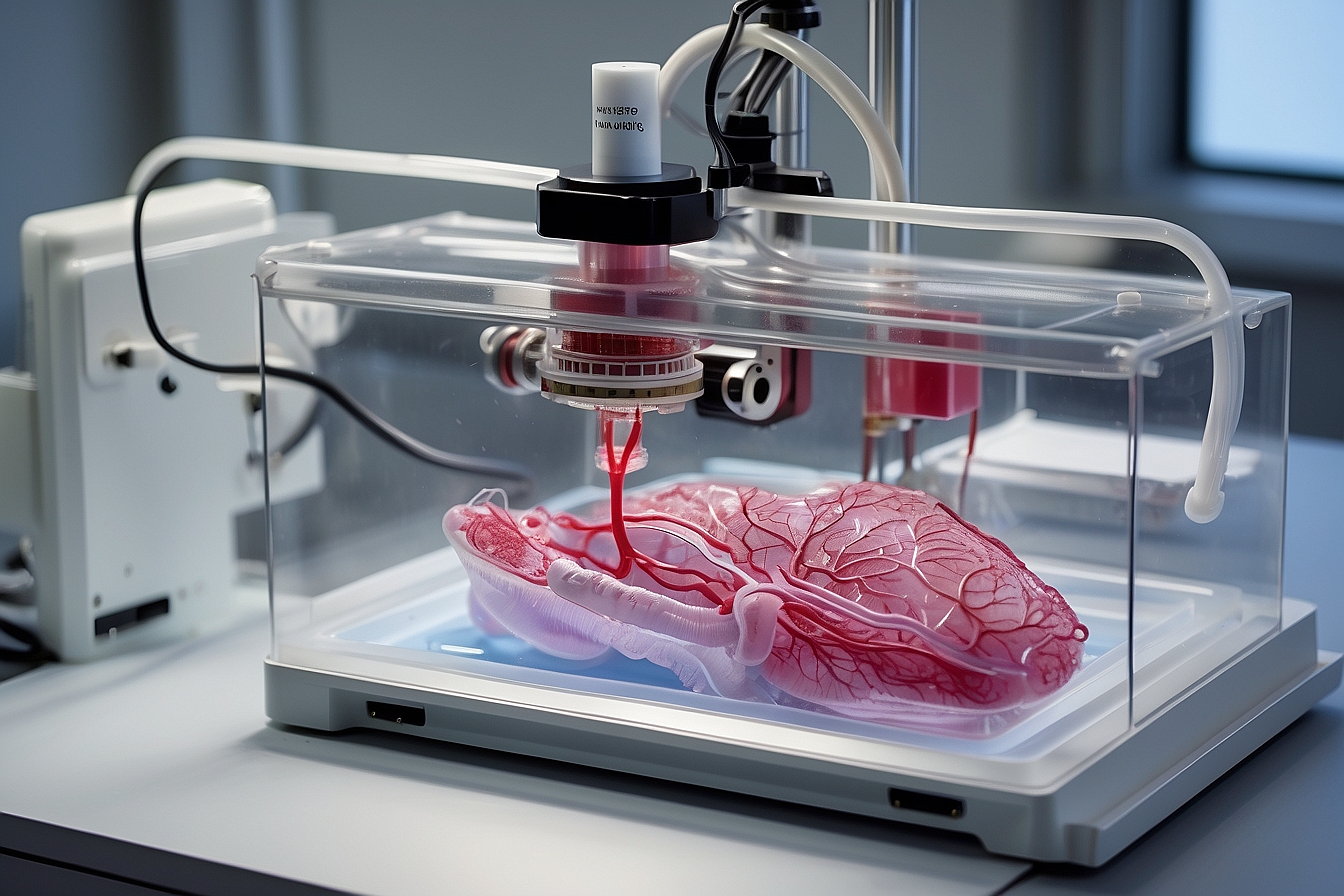
The advent of 3D bioprinting heralds a groundbreaking era in medical science, one that promises to significantly impact the future of organ transplantation and the lives of patients worldwide. By leveraging precision layering of cells and biomaterials, it is possible to construct organs layer by layer. This emerging technology offers the potential to produce bioartificial organs that closely replicate the structure and function of their natural counterparts.
The crux of this technology lies in the meticulous assembly of multiple cell types, growth factors, and biomaterials to fabricate complex tissue constructs. Integrating the intricate vascular networks essential for organ viability remains a significant challenge. However, advancements in this field aim to address the persistent shortfall in available donor organs, offering a beacon of hope for thousands of critically ill patients on transplant waiting lists.
As research and development in 3D bioprinting continue to mature, the potential for personalized and on-demand organ creation grows nearer to realization. This could lead to a transformative reduction in organ rejection rates, as organs could be tailor-made from a patient’s own cells. The implications of such personalized treatment options would extend far beyond individual patient care, with the possibility of reshaping healthcare protocols and organ donation systems across the globe.
Overview of 3D Bioprinting
3D bioprinting is transforming regenerative medicine by allowing the fabrication of complex, functional living tissues. This technology is moving towards the creation of replacement organs tailored to individual patients.
Principles of 3D Bioprinting
3D bioprinting involves a layer-by-layer method to deposit cells, biomaterials, and bioactive agents, following a predefined spatial geometry guided by computer models. This intricate process demands precise control of the mechanical and chemical properties, ensuring that the resulting structure can sustain cell viability and promote tissue formation.
Advancements in Bioprinting Technology
Significant advancements have been made in the bioprinting field, leading to the development of new techniques that provide enhanced resolution and cell viability. For example, inkjet-based, laser, and extrusion-based bioprinting methods are now employed to produce constructs with increasing complexity and precision. These advancements are inching us closer to the goal of fabricating fully functional organs for transplantation.
The Process of Bioprinting Organs
The bioprinting of organs involves complex procedures that ensure the creation of viable tissue structures. This technology employs precision and biomimicry to replicate the intricate details of human organs.
Pre-Printing Preparations
Bioprinting begins with pre-printing preparations essential for creating a blueprint of the target organ. This phase involves:
- Designing a Model: Utilizing medical imaging techniques such as MRI and CT scans, a digital 3D model of the patient-specific organ is created.
- Material Selection: Selecting the appropriate bioinks, which often comprise biopolymers and living cells, to match the mechanical and biochemical properties of the native organ.
Printing Techniques
Printing techniques are the core of bioprinting, where the organ is constructed layer by layer. The methods include:
- Inkjet Bioprinting: Depositing bioink droplets onto a substrate, suitable for creating tissue constructs with high cellular densities.
- Extrusion Bioprinting: Continuously dispensing bioink through a nozzle, effective for fabricating structurally complex tissues.
- Laser-Assisted Bioprinting: Using laser pulses to transfer biomaterials onto a receiving substrate, allowing high precision and cell viability.
A study titled Progress of 3D Bioprinting in Organ Manufacturing elaborates on these techniques.
Post-Printing Processes
After printing, the post-printing processes facilitate tissue maturation and functionality. This includes:
- Crosslinking: Often applying a UV light or chemical agents to stabilize the structure of the printed construct.
- Maturation: Culturing the organ in a bioreactor to promote cell differentiation, tissue development, and mechanical strength before implantation.
Research titled 3D Bioprinting of Human Hollow Organs provides insights into the post-printing stages necessary for creating functional organs.
Benefits of Bioprinted Organs
3D bioprinting of organs promises transformative advances in medicine. It leverages cutting-edge technologies to address critical healthcare challenges.
Customization Potential
The customization potential of bioprinted organs is a paramount benefit. Each organ can be tailored to the recipient’s specific anatomical and cellular makeup, potentially reducing the risk of rejection. This precision is achieved by using the patient’s own cells, ensuring compatibility and a custom fit.
Reduction in Organ Shortage
Bioprinted organs have the potential to significantly reduce the organ shortage crisis. By creating organs on-demand, patients may no longer have to endure long waiting periods for donor organs, which can sometimes lead to worsened conditions or death. This advancement could also decrease reliance on organ transplants from donors.
Ethical Advantages
The ethical advantages of bioprinted organs are notable. Since they are created from the patient’s own cells, issues surrounding organ donation and transplantation ethics, such as the illegal organ trade and donor consent, are vastly mitigated.
Challenges in 3D Bioprinting
While 3D bioprinting of organs harbors the potential to revolutionize transplant medicine, several formidable challenges need to be addressed. These hurdles span technical, biological, and regulatory domains.
Technical Hurdles
Technical challenges in 3D bioprinting include the resolution and precision of the printers themselves. Achieving the intricate detail necessary to replicate the complex architecture of human organs is a significant obstacle. Moreover, there is a need for advanced biomanufacturing technologies that can support the development of organs with viable structural integrity and function.
Biocompatibility Concerns
The materials used in 3D bioprinting must be biocompatible to avoid rejection by the recipient’s immune system. Balancing scaffold strength with the promotion of cell growth and differentiation is critical. Key to success is the development of materials that can support cell viability while degrading at a rate that matches the tissue regeneration process.
Regulatory and Ethical Issues
The advent of 3D-printed organs faces a myriad of regulatory and ethical questions. Regulatory bodies must establish guidelines for the manufacture and use of these organs to ensure they are safe and effective. Furthermore, ethical considerations concerning distributive justice and the potential for bioprinting to be used for non-therapeutic enhancements must be thoroughly debated and addressed.
Current State of 3D Bioprinting
3D bioprinting is a transformative technology, leveraging precision-driven automation to create cellular constructs that emulate the complexity of human tissues and organs. At its core, the technology has evolved to print biological constructs with a level of intricacy akin to native tissue structures. Pioneers in the field are combining biopolymers and stem cells, or ‘bioinks,’ to develop living functional tissues, charting a course towards addressing the chronic shortage in tissue replacement and organ transplantation.
Current bioprinting methods include inkjet, laser, and pressure-based techniques. Inkjet bioprinting is one of the most widely adopted due to its cost-efficacy and accessibility. Laser-assisted bioprinting, while more expensive, provides higher resolution. Pressure-based methods, which include pneumatic and microvalve systems, offer robust versatility for a range of bioinks.
| Method | Features |
|---|---|
| Inkjet | Low-cost, accessible, adaptable |
| Laser-assisted | High resolution, precise |
| Pressure-based | Versatile, compatible with a variety of bioinks |
Given the complexity of human organs, the 3D bioprinting field currently focusses on fabricating simpler tissue structures for drug screening and disease models. While printing whole organs is a goal on the horizon, it presents challenges in vascularization and cell viability over larger scales.
Bioprinted tissues have made significant strides in fields such as dermatology, where skin models are essential for cosmetic testing. Research continues into the bioprinting of hollow organs, such as bladders and blood vessels, which are less complex than solid organs. Overall, 3D bioprinting stands as a promising beacon for the future of regenerative medicine, with ongoing advancements heralding a new era in medical science and patient care.
Future Prospects
The landscape of organ transplantation is poised for transformation as 3D bioprinting advances towards creating viable tissues and organs for clinical use. These technologies converge at the intersection of research, patient care, and long-term healthcare strategies.
Research and Development
In research and development, 3D bioprinting is continually evolving. Currently, it hinges on the precise assembly of cells and biomaterials to replicate organ function. Pioneering studies have yielded 3D bioprinted tissue that opens pathways for novel treatment methods and offers a foundation for future breakthroughs.
Clinical Applications
Regarding clinical applications, the main goal is the production of organ substitutes that are less prone to rejection, as they can be created from the patient’s own cells. It’s anticipated that 3D bioprinting can potentially solve the organ donor crisis, by providing custom tissues and organs, thus drastically reducing transplant waitlists and saving countless lives.
Long-Term Implications
The long-term implications of 3D bioprinting stretch beyond individual patient care. They may reshape healthcare systems by reducing the costs associated with organ transplants and chronic diseases. By deploying bioartificial organs, it could promote greater longevity and significantly elevate the quality of life for those requiring transplants.
Impact on Healthcare
3D bioprinting of organs holds the potential to dramatically enhance patient survival rates and reduce healthcare costs by minimizing the dependence on organ donors.
Patient Outcomes
The advent of 3D bioprinting technology has the potential to improve patient outcomes significantly. Customized organs printed to match patient-specific anatomies can lead to a better fit, function, and reduced risk of rejection. As the technology advances, it also holds the promise of reducing waiting times for transplants, thereby saving more lives.
Healthcare Economics
Economically, the ability to bioprint organs could have a considerable impact by alleviating the costs associated with long-term care for patients awaiting transplants. Simplifying the supply chain for organ procurement, this technology could reduce overall expenditures and, in the long term, make transplants more accessible, even potentially streamlining the process enough to make preventative bioprinting a feasible option.
Frequently Asked Questions
The advent of 3D bioprinting has opened up new frontiers in medical science, particularly in the realm of organ transplants, prompting several common inquiries about its capabilities and future.
What is the current state of success in 3D printed organ transplants?
Although not yet applied in standard clinical settings, 3D printed organs have shown promise in small-scale studies and under laboratory conditions. Efforts continue to improve the function and reliability of these bioprinted organs for eventual human application.
How do 3D printed organs integrate with human biology?
Integration of 3D printed organs with human biology relies on the precise arrangement of various cell types and the inclusion of growth factors to encourage tissue growth and organ functionality post-transplant.
What role do stem cells play in 3D printing organs?
Stem cells are pivotal in the bioprinting process because of their ability to differentiate into diverse cell types required for creating complex tissues, thus forming the basis for constructing the bioartificial organs.
What prospective benefits does 3D bioprinting offer to regenerative medicine?
Bioprinting contributes enormously to regenerative medicine by potentially providing tailor-made organs that could reduce transplant rejections and eliminate the shortage of donor organs.
When is it anticipated that 3D printing of organs will become a standard medical procedure?
While it’s difficult to predict a precise timeline, advancements in the field suggest that 3D printing of organs could be embraced as a standard procedure within the coming decades as technology matures and regulatory hurdles are overcome.
How might 3D bioprinting transform the landscape of organ transplantation?
The ability to produce organs on demand could substantially diminish waiting lists for transplants and enhance the success rates by providing more biocompatible options for patients.